Target-OXY
Towards Targeted Oxytocin Treatment in Alcohol Addiction (Target-OXY)
Worldwide two billion people drink regularly alcohol. A major health consequence is alcohol addiction that is characterized by chronic relapses. Preventing relapse is the main treatment goal. Current pharmacological treatments have limited effectiveness and there is a large het-erogeneity in the treatment response. Better treatments and prediction approaches that can be easily translated into the clinical situation are warranted. In our e:Med funded SysMedAlco-holism consortium we have identified early warning signs and drinking profiles that predict future relapse behavior and treatment response of clinically used anti-relapse medications. We have also identified in a multi-omics approach alterations in the oxytocin (OXY) system in alcoholic patients suggesting OXY as a candidate medication to reduce relapse. Here our goals are (i) to demonstrate the clinical applicability of OXY and (ii) to computationally predict relapse and identify treatment responsive individuals. For the demonstration of the clinical applicability of OXY, we propose a new module in the drug development process, namely a preclinical multicenter placebo controlled trial in rats with a step-wise transla-tion into a naturalistic pilot trial with ambulatory assessment in alcoholic patients. As a comparator, we will use datasets from previous trials where we tested placebo vs acampro-sate – which is a clinically effective medication. These data will be contrasted with a 3-arm design in male and female alcohol addicted rats where two doses of intranasally applied OXY will be tested against placebo in a well-validated rat model for alcohol addiction. From this pre-clinical trial we will obtain intensive longitudinal data (ILD) sets on drinking and activity. Us-ing new in silico approaches we will then be able to identify early warnings signs and drink-ing clusters for relapse and OXY treatment responsive individuals. This preclinical work will guide our naturalistic trial with ambulatory assessment in alcoholic patients.
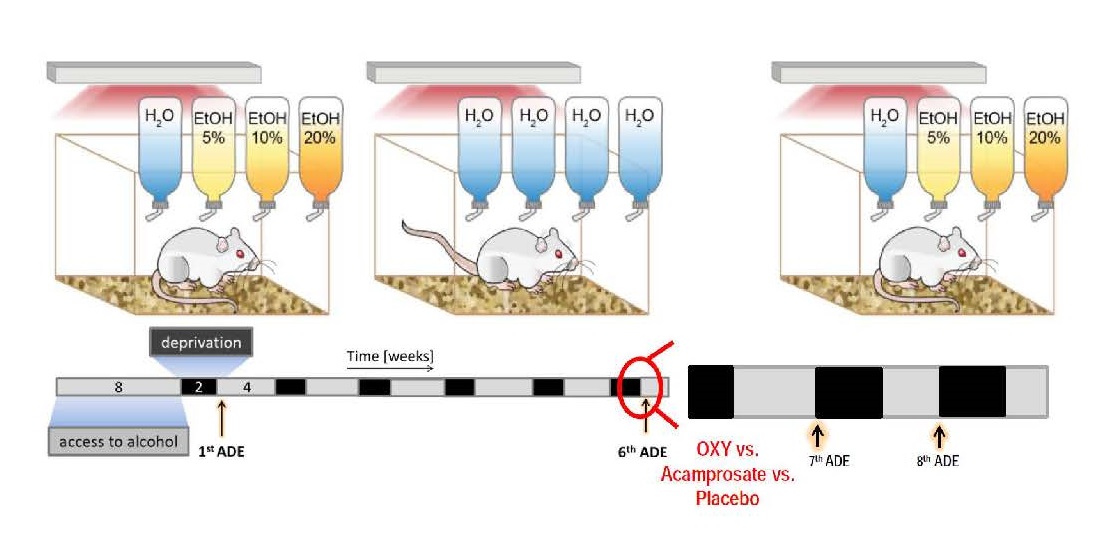
Fig. Experimental design and time line for the preclinical trial. In the upper row the free choice 4-bottle paradigm (with water, 5, 10 and 20% ethanol solutions) in the DRINKOMETER system is illustrated. The timeline of the experimental approach is shown below – all rats will have access to ethanol for 8 weeks (grey bar) followed by two weeks of deprivation (black bar) and then again 4 weeks of alcohol access etc. By the 6th ADE rats show compulsive relapse behavior and drug testing with OXY vs placebo starts. Possi-ble long-lasting effects will be studied during the 7th and 8th ADE (at both time points no drug intervention will be made). As a comparator we will use our existing database on acamprosate treatment.



