SP 5
Analysis of peripheral tolerance signature early after transplantation to identify low risk patients
We could previously show that operational tolerant kidney transplant patients are characterized by a specific peripheral transcription profile associated with increased levels of non-activated B cells at later stages after transplantation.
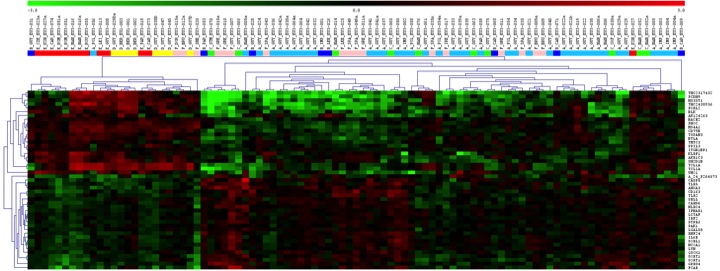
mRNA-Microarray analysis of whole blood samples from 1) operationally tolerant patients without immunosuppression (red squares), 2) quasi tolerant patients receiving only low-dose steroids (light red squares), 3) stable stable patients receiving calcineurin inhibitor -CNI -based immunosuppression (light blue squares), 4) stable patients patients receiving CNI-free immunosuppression, 5) chronically rejecting patients (green squares) and 6) helathy controls (yellow squares). Total RNA of blood samples collected into Paxgene tubes was isolated and reverse transcribed and subjected to Microarray analyses using whole genome Agilent Arrays.
Further preliminary analysis revealed that some patients alt-hough on immunosuppression develop this signature within the first year. Thus, it seems likely that the tolerance signature can be used for an early treatment optimization of kidney transplant patients.
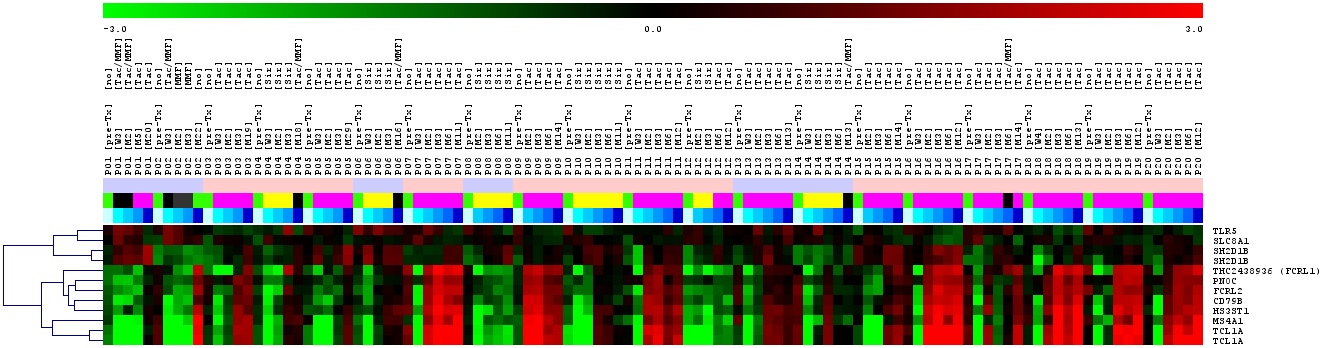
Within e:Kid we will generate an RNA/cDNA biobank containing over 3.000 samples from kidney transplant patients. Furthermore, we will perform a qPCR profiling of those samples concentrating mainly on the described “tolerance signature” but also other previously identified gene markers with potential relevance for transplant outcome such as TCAIM or Foxp3. The generated data will be associated with clinical outcome to predict individual immune reactivity. Very importantly, biomarkers obtained by statistical analysis and mathematical modeling with the help of other project partners will be used for a subsequent prospective study on personalized immunosuppressive therapy in the second funding period.
Keywords: Transplantation, Tolerance, mRNA Expression, B cells, T cells



